In Addition to the Main Product, What Are Two Side Reactions That Could Occur in This Experiment?
13.12: Outcome of Calculation a Reactant or Product
- Page ID
- 49533
If we accept a system which is already in equilibrium, addition of an actress amount of ane of the reactants or i of the products throws the system out of equilibrium. Either the forrad or the opposite reaction will so occur in order to restore equilibrium atmospheric condition. We can easily tell which of these two possibilities will happen from Le Chatelier's principle. If we add together more of ane of the products, the system will suit in order to offset the proceeds in concentration of this component. The reverse reaction will occur to a limited extent so that some of the added product can exist consumed. Conversely, if i of the reactants is added, the organization will arrange past allowing the forward reaction to occur to some extent. In either instance some of the added component will be consumed.
We come across this principle in operation in the example of the decomposition of Hello at loftier temperatures:
\[\text{2HI}(k) \rightleftharpoons \text{H}_2(g) + \text{I}_2(yard)\]
The addition of H2 has increased the concentration of this component. Accordingly, Le Chatelier's principle predicts that the system volition attain a new equilibrium in such a way every bit to reduce this concentration. The reverse reaction occurs to a limited extent. This not only reduces the concentration of H2 but the concentration of I2 as well. At the same fourth dimension the concentration of HI is increased. The organisation finally ends upward with the concentrations calculated in Example two from Calculating the Extent of a Reaction, namely,
\[\frac{\text{ }\!\![\!\!\text{ H}_{\text{2}}]\text{ }\!\!]\!\!\text{ }\!\![\!\!\text{ I}_{\text{ii}}\text{ }\!\!]\!\!\text{ }}{\text{ }\!\![\!\!\text{ Hullo }\!\!]\!\!\text{ }^{\text{2}}}=\frac{\text{0}\text{.1018 mol L}^{-\text{1}}\text{ }\times \text{ 0}\text{.001 82 mol L}^{-\text{1}}}{\text{(0}\text{.0963 mol L}^{-\text{ane}}\text{)}^{\text{2}}\text{ }}=\text{0}\text{.02}=K_{c}\]
The style in which this system responds to the addition of H2 is also illustrated schematically in Figure i. The actual extent of the change is exaggerated in this figure for diagrammatic effect.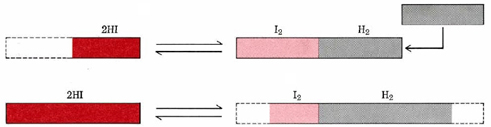
Le Chatelier'southward principle can as well be applied to cases where one of the components is removed. In such a case the system responds past producing more of the component removed. Consider, for example, the ionization of the weak diprotic acid H2S:
\[\ce{H_{ii}S + 2H_{ii}O \rightleftharpoons 2H_{iii}O^{+} + S^{ii-}}\]
Since HiiS is a weak acid, very few Sii– ions are produced, merely a much larger concentration of Southtwo–ions can exist obtained by adding a strong base. The base will consume most of the H3O+ ions. Equally a result, more H2S will react with HiiO in order to make upwards the deficiency of H3O+, and more Southii–ions will also be produced. This fox of removing one of the products in order to increase the concentration of another product is ofttimes used by chemists, and also by living systems.
Previously, we've investigated the effect of calculation/subtracting a product/reactant in mathematical terms. The video below allows you lot to visually see the changes the equilibrium shifts that occur upon the addition of a reactant or product, courtesy of the N Caroline School of Science and Mathematics.
Example \(\PageIndex{i}\) : Yield
When a mixture of i mol Nii and 3 mol Htwo is brought to equilibrium over a catalyst at 773 Chiliad (500°C) and 10 atm (one.01 MPa), the mixture reacts to form NH3 according to the equation
\(\text{N}_2(thousand) + \text{3H}_2(g) \rightleftharpoons \text{2NH}_3(thousand)\) ΔHm = – 94.3 kJ The yield of NHiii, however, is quite pocket-sized; merely about two.v percent of the reactants are converted. Advise how this yield could exist improved (a) by altering the pressure level; (b) by altering the temperature; (c) past removing a component; (d) by finding a better catalyst.
Solution:
a) Increasing the pressure will drive the reaction in the direction of fewer molecules. Since Δn = - two, the frontward reaction will be encouraged, increasing the yield of NH3. b) Increasing the temperature will drive the reaction in an endothermic management, in this example in the reverse management. In guild to increment the yield, therefore, we need to lower the temperature. c) Removing the production NH3 will shift the reaction to the right. This is usually done by cooling the reaction mixture so that NHiii(l) condenses out. Then more Northward2(1000) and H2(g) are added, and the reaction mixture is recycled to a condition of sufficiently high temperature that the charge per unit becomes appreciable. d) While a better catalyst would speed upward the attainment of equilibrium, it would non affect the position of equilibrium. It would therefore accept no event on the yield.
Note: As mentioned in Chaps. 3 and 12, NHthree is an of import chemical considering of its utilise in fertilizers. In the design of a Haber-process establish to industry ammonia, attempts are made to use as high a force per unit area and every bit low a temperature as possible. The pressure is usually of the order of 150 atm (15 MPa), while the temperature is not normally below 750 K. Although a lower temperature would give a higher yield, the reaction would go besides slowly to exist economical, at least with present-day catalysts. The discoverer of a amend catalyst for this reaction would certainly become a millionaire over-night.
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Book:_ChemPRIME_%28Moore_et_al.%29/13:_Chemical_Equilibrium/13.12:_Effect_of_Adding_a_Reactant_or_Product

0 Response to "In Addition to the Main Product, What Are Two Side Reactions That Could Occur in This Experiment?"
Post a Comment